Arxius de Miscel·lània Zoològica. Volumen 23 (2025) Páginas: 51-61
Evaluation of the entomological biodiversity of the Maamora forest
Habbaz, H., Maatouf, N., Rohi, L.
DOI: https://doi.org/10.32800/amz.2025.23.0051Descargar
PDFCita
Habbaz, H., Maatouf, N., Rohi, L., 2025. Evaluation of the entomological biodiversity of the Maamora forest. Arxius de Miscel·lània Zoològica, 23: 51-61, DOI: https://doi.org/10.32800/amz.2025.23.0051-
Fecha de recepción:
- 23/10/2024
-
Fecha de aceptación:
- 21/02/2025
-
Fecha de publicación:
- 26/03/2025
-
Compartir
-


-
Visitas
- 1057
-
Descargas
- 363
Abstract
Evaluation of the entomological biodiversity of the Maamora forest
This study aimed to explore and evaluate the entomological biodiversity of the Maamora forest through a structural study of the coleopteran population, which contributes to the proper functioning of this ecosystem. It is an inventory at the level of the cork oak forest of Maamora that took place over two consecutive years (2021 and 2022) using different types of traps and various sampling techniques. This inventory identified 17 orders of Arthropods. The order of Coleoptera is represented by 256 species, belonging to 42 families, subdivided into eight functional groups according to their diets. The calculated ecological indices confirm the significant diversity of the environment, with the Shannon index being greater than 4 and the Simpson index not exceeding 0.16. These results have complemented and updated the work of other studies conducted in the region and will fill the gap in information on beetles in the cork oak forests of Morocco. The presence of such insect biodiversity in the Maamora cork oak forest is an indicator of the important heritage value of the environment, which calls on managers and decision-makers to protect it from the threats that weigh on this environment.
Dataset published through GBIF (DOI: 10.15470/0z8ret)
Key words: Inventory, Biodiversity, Beetles, Forest, Cork oak, Maamora, Morocco
Resumen
Evaluación de la biodiversidad entomológica en el bosque de Maamora
Este estudio pretende explorar y poner en valor la biodiversidad entomológica del bosque de Maamora mediante un estudio estructural de la población de coleópteros, que contribuye al buen funcionamiento de este ecosistema. Se trata de un inventario del bosque de alcornoque de Maamora que se realizó durante dos años consecutivos (2021 y 2022), mediante varias clases de trampas y varios métodos de muestreo. Este inventario identificó 17 órdenes de Arthropoda. El orden Coleoptera está representado por 256 especies, pertenecientes a 42 familias, subdivididas en ocho grupos funcionales según su dieta. Los índices ecológicos calculados confirman la importante diversidad del entorno, de forma que el índice de Shannon es superior a 4 y el índice de Simpson no pasa de 0,16. Estos resultados complementan y actualizan el trabajo de otros estudios realizados en la región y permitirán llenar el vacío de información que existe sobre los escarabajos que viven en los bosques de alcornoque de Marruecos. La biodiversidad de insectos en el bosque de alcornoque de Maamora es un indicador del importante valor patrimonial de este entorno, y es un aviso para que las personas que gestionan el territorio y toman decisiones lo protejan de los peligros que lo amenazan.
Datos publicados en GBIF (DOI: 10.15470/0z8ret)
Palabras clave: Inventario, Biodiversidad, Escarabajos, Bosque, Alcornoque, Maamora, Marruecos
Resum
Avaluació de la biodiversitat entomològica al bosc de Maamora
Aquest estudi pretén explorar i donar valor a la biodiversitat entomològica del bosc de Maamora mitjançant un estudi estructural de la població de coleòpters, la qual contribueix al bon funcionament d'aquest ecosistema. Es tracta d'un inventari del bosc d'alzina surera de Maamora que es va dur a terme durant dos anys consecutius (2021 i 2022), mitjançant diverses classes de trampes i diversos mètodes de mostreig. L'inventari va identificar 17 ordres d'Arthropoda. L'ordre Coleoptera està representat per 256 espècies, pertanyents a 42 famílies, subdividides en vuit grups funcionals segons la dieta. Els índexs ecològics calculats confirmen la important diversitat de l'entorn, de manera que l'índex de Shannon és superior a 4 i l'índex de Simpson no passa de 0,16. Aquests resultats complementen i actualitzen el treball d'altres estudis duts a terme a la regió, i permetran omplir el buit d'informació que hi ha pel que fa als escarabats que viuen als boscos d'alzina surera del Marroc. La biodiversitat d'insectes al bosc d'alzina surera de Maamora és un indicador de l'important valor patrimonial d'aquest entorn, i és una crida a les persones que gestionen el territori i prenen decisions perquè el protegeixin dels perills que l'amenacen.
Dades publicades a GBIF (DOI: 10.15470/0z8ret)
Paraules clau: Inventari, Biodiversitat, Escarabats, Bosc, Alzina surera, Maamora, Marroc
Introduction
Forests cover nearly 31 % of the Earth's land surface and are home to 80 % of the planet's terrestrial biodiversity (FAO 2020). Of the estimated 8 million species on Earth, 75 % are insects (IPBES 2019). These forest ecosystems provide essential habitats for numerous plant and animal species (Shvidenko et al 2005). However, anthropogenic pressures and the intensification of extreme weather events are causing an alarming decline in global forest cover. This deforestation fragments and destroys natural habitats, negatively impacting biodiversity and the ecosystem services upon which human societies depend.
Forests are home to a multitude of essential dendro-microhabitats, providing shelter, breeding sites, and food sources for numerous specialized species. Among these, certain insect groups play a crucial ecological role in maintaining the balance and health of forest ecosystems (Noriega et al 2018). These insects ensure natural population regulation, are essential for pollinating 75 % of our crops, and contribute to the recycling of organic matter, improving soil quality. As a result, they are excellent bioindicators of the conservation status and health of forest ecosystems (Marage et al 2017).
Insect biodiversity is an invaluable natural heritage. However, in recent years, populations have undergone an alarming decline due to the loss and fragmentation of semi-natural habitats, primarily caused by deforestation (Jactel et al 2021). Beetles are severely affected by anthropogenic disturbances due to their high habitat quality requirements (Loukou et al 2017). Insects thus serve as excellent bioindicators for assessing the ecological status of forest ecosystems and the effectiveness of conservation management practices. Before implementing conservation measures aimed at protecting iconic species and various functional groups, it is essential to conduct targeted inventories of certain insect groups considered as bioindicators.
Moroccan forests harbor a remarkable biodiversity. Among the 24,000 animal and 7,000 plant species recorded, 1,700 are considered rare or threatened, and have a high endemism rate, this being 11 % for fauna and over 20 % for vascular flora (ONEDD 2015). The Maamora forest, dominated by cork oak Quercus suber L., 1753 and plantations of eucalyptus, acacia, and pine, is an emblematic example. Considered the largest contiguous cork oak forest in the world (Natividade 1956), it represents 17 % of the total area of cork oak forests in Morocco and 25 % of those in the Atlantic forests (Aafi 2007).
The Maamora forest harbors a particularly rich vascular flora, representing 48 % of the flora of Moroccan cork oak forests (Sauvage 1961) and 9.3 % of the national flora (Benabid 2000). This significant botanical diversity is accompanied by an equally remarkable fauna, with over 700 species of arthropods (Villemant and Fraval 1991) and 150 species of birds (Cherkaoui et al 2007). These biological assets have earned the Maamora recognition as a Site of Biological and Ecological Interest (SIBE) by the study on Protected Areas in Morocco (AEFCS 1996).
Knowledge of the status and trends of beetle species in Morocco is limited. Moreover, there is no comprehensive list of rare or threatened species, especially in the context of climate change affecting forest ecosystems that are already fragile due to human activities. Therefore, it is urgent to conduct a status assessment of the Moroccan entomofauna to characterize insect communities present in sensitive natural areas and to determine priorities for the conservation and restoration of these environments.
To gain deeper insight into the diversity, abundance, and ecological role of beetles in the Maamora forest, we conducted an inventory over two consecutive years (2021 and 2022). Passive and active sampling methods were combined along linear transects within the cork oak forest. The primary objective of this study was to assess the ecological status of the Maamora forest with a view to implementing sustainable management strategies aimed at preserving the functional biodiversity of Moroccan cork oak forests.
Materials and methods
Study area
The Maamora forest is located between the cities of Salé and Kénitra, along the Atlantic coast in north-west Morocco (fig. 1). It extends eastwards along an area of 40 km wide and 70 km long, following a bioclimatic gradient ranging from sub-humid to semi-arid (Belghazi and Mounir 2016). This forest covers approximately 135,000 hectares, including 71,000 hectares of commercial plantations and 64,461 hectares of cork oak woodlands (Belghazi and Mounir 2016), representing 17 % of the total area of cork oak forests in Morocco and 25 % of the area of Atlantic cork oak forests (Aafi 2007).

Fig. 1. Maamora forest in Morocco and location of the studied stations (S1, S2, S3).
Fig. 1. El bosque de Maamora, en Marruecos, y ubicación de las zonas de estudio (S1, S2, S3).
The topography of the forest is mostly flat, interrupted by a southwest-northeast oriented hydrographic network that divides it into five distinct Cantons named A, B, C, D, and E, respectively, and distributed from sea level up to 300 m a.s.l.
The climate is of the Mediterranean type, tempered by the influence of the Atlantic Ocean. The average temperatures range between 1.8 ºC in January (year 2005) and 38 ºC in August (year 2012), with annual rainfall ranging from 403 to 557 mm, according to years. Rains are generally concentrated in November, December, and January, but their distribution can vary from year to year. The dry period is relatively long, lasting up to 6 months, but atmospheric humidity is high, especially in the western part, which partly compensates for the aridity of the climate. Climatic data classify the western sectors of the Maamora forest as 'warm sub-humid bioclimate' and the eastern sectors as 'temperate semi-arid bioclimate' (Belghazi and Mounir, 2016).
The natural vegetation of the Maamora consists mainly of cork oak trees Quercus suber L. and a few specimens of the Maamora pear Pyrus mamorensis Trabut, an endemic species to the region. Mastic trees Pistacia lentiscus L., wild olive Olea europaea ssp. oleaster, and green olive trees or mock privet Phillyrea latifolia L. are found on soils with shallow sand and red soil. The dwarf palm, Chamaerops humilis L., grows in dense clumps in the more open areas. Phoenician juniper Juniperus phoenicea L. can be found along the Atlantic coast, particularly around Lake Boughaba. The shrub and herb communities are highly diverse, with 402 recorded species (Metro and Sauvage 1955, Sauvage 1961, Aafi 2007). The parts of the forest which have been impacted by commercial plantations are covered with introduced species: pine (Pinus pinaster ssp., atlantica H. del Vill., Pinus halepensis Mill., and Pinus pinea L.), eucalyptus (mostly Eucalyptus camaldulensis Dehn), and black wattle (or tannin acacia) trees (Acacia mearnsii De Wild.).
Collection sites
This study was conducted in three stations distributed across three cantons (A, C, and E) within the forest, chosen based on their distance from the sea, the degree of enclosure of the plots, the bioclimatic zone, the age of the cork oak trees, and the density of reforestation with exotic species (fig. 1). Fencing prevents pruning and wood cutting by the local population and prevents cattle grazing in the undergrowth, which preserves the herbaceous and low shrub cover.
• Station S1 (Canton A): located in the western part of the forest (34º 12' 33.34'' N, 06º 35' 54.78'' W), at an elevation of 37 m belongs to the warm sub-humid bioclimate with maritime influences. This station was characterized by an open cork oak forest where cork oak was mixed with tannin acacia, within a semi-fenced plot.
• Station S2 (Canton C): located in the middle part of the forest (34º 04' 57.73'' N, 06º 24' 38.31'' W), at an elevation of 134 m belongs to the warm sub-humid bioclimate with maritime influences. This plot of old cork oak trees with a very dense understory was completely fenced and thus, protected from grazing.
• Station S3 (Canton E): located in the eastern part of the forest (34º 09' 36.1'' N, 06º 07' 04.34'' W), at an elevation of 164 m belongs to the semiarid bioclimate with temperate winters and continental influences. This unfenced station comprised a mixed cork oak forest with eucalyptus trees.
The material collected was deposited in the insect collection of the Centre for Innovation, Research, and Training, Rabat (Morocco).
Sampling methods
Several sampling methods were used: visual capture when checking the traps; and passive sampling systems with three types of traps:
• Window trapping (interception traps): the traps consisted of a large transparent plexiglass panel (73 cm x 42 cm) that intercepted the beetles that struck it in flight. The insects were collected in a gutter fixed at the base of the trap. This gutter was filled with water and mixed with saline solution and a few drops of dishwashing detergent to reduce surface tension. This trapping method is effective for collecting xylophagous and saproxylic species (Ranius and Jansson 2002, Brustel 2004, Bouget et al 2008). Their position was 1.2 m from the ground, situated in plots where dead wood was present (Bouget and Noblecourt 2005).
• Pitfall traps (Barber traps): these traps were made with plastic cups with a top diameter of 60 mm. They were placed with the opening at ground level, and covered with wooden branches to prevent trampling by animals. Pitfall traps allow for the capture of soil-dwelling invertebrates (Nageleisen and Bouget 2009). The distance between the traps was 20 metres.
• Coloured bowls: these traps consisted of coloured bowls (yellow, orange, white, and blue) with a diameter of 15 cm and a height of 13 cm. They were filled halfway with a preservation mixture (soapy water + salt) and placed at a height of 1.5 m above ground level, in cork oak clearings to attract species attracted to flowers.
The traps were installed at three stations in Cantons A, C, and E. At each station, 13 traps were set up at a distance ranging from 15-20 m apart (eight barber traps, four coloured traps, with one each of yellow, white, orange, and blue, and one window trap). Traps were checked every three weeks for a 7-month period (April to October) during two consecutive seasons (2021 and 2022). The specimens collected were first identified to the genus level using specific identification keys for each family.
Shannon-Wiener Index (H')
Used to assess the diversity of a community and is considered the best way to measure diversity (Dajoz 2008). Its formula is as follows:
H' = 3 pi ln pi
where S corresponds to the number of taxa collected and reflects the diversity of a sample; and pi corresponds to the proportion of species i relative to the total number of species (S) in the study area, calculated as pi = ni / N
This index allowed us to quantify the heterogeneity of biodiversity in a study environment, and therefore observe its evolution over time. This index ranged from 0 to ln S. The higher the value of the H' index, the greater the diversity.
Simpson's Index (D)
Gives more weight to the most frequent species than to the total species richness. Its formula is:
D = 3 pi2
This index always ranges from 0 to 1. Specific diversity is highest when the Simpson index is lowest, close to 0, whereas biodiversity is lowest when the value is close to 1.
Evenness (E)
The relative abundance structures of species determine evenness or the dominance component of diversity. The measure of evenness corresponding to the Shannon-Weaver index was calculated using the following formula:
E = H' / log2 S
Jaccard Index (Ji+j)
It considers the presence or absence of species. It allows us to highlight the similarities or differences (between populations) that have the greatest influence on the distribution of species between stations. This coefficient is the ratio, expressed as a percentage, between the species common to both stations and the total number of species present in these stations. It is expressed as follows:
Ji+j = a / (a + b + c) x 100
The values of the Jaccard index range from 0 to 100. The closer the values are to 100, the more qualitatively similar the two populations are.
Trophic niche of the species
All captured beetles were identified to the genus level using various identification keys. The diet of each species was ascertained by referring to the works of (Velle 2004, Bouget et al 2005, Brin and Brustel 2006).
Results
A two-year trapping study at the three sites revealed 17 orders of Arthropods (see the dataset published through GBIF with DOI: 10.15470/0z8ret). Figure 2 shows the relative abundance of each order.
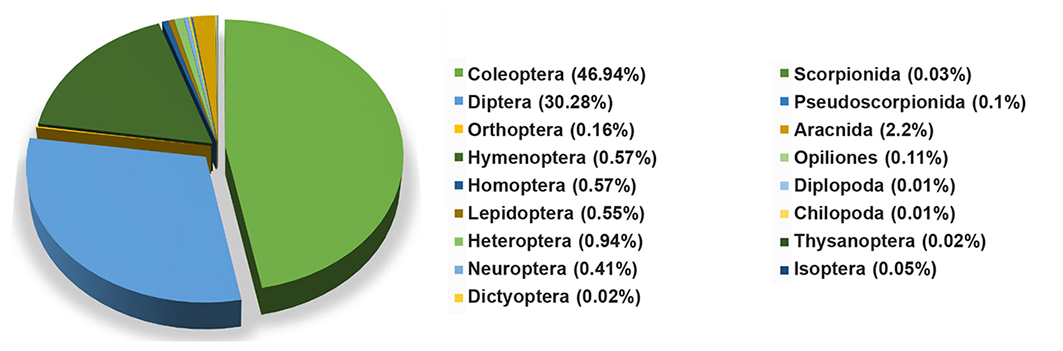
Fig. 2. Relative abundance of different arthropod orders trapped in the Maamora cork oak forest.
Fig. 2. Abundancia relativa de diferentes órdenes de artrópodos capturados en el bosque de alcornoque de Maamora.
The Coleoptera community constitutes nearly 47 % of the recorded specimens, belonging to 42 families. Figure 3 illustrates the relative abundance of each of the 42 families captured, across all sampled sites over the two-year study period. Details concerning the species and their distribution across the stations are provided in the appendix.

Fig. 3. Distribution of the abundances (%) of families sampled in the Maamora cork oak forest.
Fig. 3. Distribución de las abundancias (%) de las familias capturadas en el bosque de alcornoque de Maamora.

Appendix 1. Species abundance according to sampling sites: S1, Canton A; S2, Canton C; and S3, Canton E.
Appendix 1. Species abundance according to sampling sites: S1, Canton A; S2, Canton C; and S3, Canton E.
The most represented families were: Tenebrionidae (27 %), Meloidae (15 %), Glaphyridae (10 %) and Buprestidae (9 %), representing 61 % of the captured specimens.
In terms of species richness, the Tenebrionidae and Carabidae families were the richest with 27 species each, followed by Curculionidae, Scarabaeidae, Chrysomelidae, Buprestidae and Dermestidae with respectively 24, 21, 18, 14 and 13 species. While 12 families were represented by two species and 8 families were each represented by only one taxon (fig. 4).

Fig. 4. Species richness per family of Coleoptera in the Maamora forest.
Fig. 4. Riqueza de especies por familia de Coleoptera en el bosque de Maamora.
The results of this study identified 256 species belonging to 42 taxonomic families. An analysis of the trophic organization of the captured Coleoptera revealed the presence of several functional groups. Saproxylophagous species, which depend on dead wood, accounted for 24 % of the captured species. Other ecological groups were relatively less represented: phytophagous (22 %), zoophagous (18 %), and anthophagous (16 %). Necrophagous, coprophagous, xylophagous, and mycophagous species represented less than 20 %.
In terms of abundance, saproxylophagous and anthophagous species each accounted for 41%, totalling 82 % of the overall population. Zoophages followed with 6 %. However, the other species were present in much smaller proportions: necrophages (5 %), phytophagous (3.5 %), coprophages (2 %), xylophages (1 %), and mycophagous (0.5 %) (fig. 5).

Fig. 5. Variation of abundance and specific richness of functional groups in the Maamora forest.
Fig. 5. Variación de la abundancia i la riqueza específica de grupos funcionales en el bosque de Maamora.
Seasonal patterns in beetle captures were observed, with peaks in March, April, and May of 2021, and a slight shift to April, May, and June in 2022. Figure 6 displays monthly beetle captures by trap type.

Fig. 6. Evaluation of the total abundance of Coleoptera (N, number of specimens captured) during the two years of capture.
Fig. 6. Evaluación de la abundancia total de Coleoptera (N, número de especímenes capturados) durante los dos años de capturas.
Ecological indices
The results of the calculation of the Shannon (H'), Simpson (D) indices and the relative equitability (E%) for each index in the three stations are presented in table 1.

Table 1. Variation of ecological diversity and equitability indices according to the surveyed stations.
Table 1. Variation of ecological diversity and equitability indices according to the surveyed stations.
Our results revealed high levels of biodiversity in all three study sites, as indicated by high Shannon diversity indices and low Simpson dominance indices. Furthermore, evenness values suggest a relatively balanced species distribution.
To compare the species composition of the three stations, Jaccard similarity indices were calculated. Results are shown in table 2.

Table 2. Jaccard similarity indices among stations.
Table 2. Jaccard similarity indices among stations.
The Jaccard similarity index revealed moderate similarity among the three stations. The pair with the lowest similarity was Stations 1 and 2.
The analysis of the organization of functional groups within the Coleoptera population showed unstable dynamics across the three study sites due to various biotic and abiotic factors. Figure 7 shows the distribution of species according to their trophic level at the studied sites.

Fig. 7. Distribution of beetle functional groups across three habitats.
Fig. 7. Distribución de los grupos funcionales de escarabajos en los tres hábitats.
Discussion
Our study of the Maamora forest entomofauna revealed a total of 15,201 specimens across 17 orders, exceeding previous records of 10 and 8 orders (El Alami Idrissi 2013, Villemant and Fraval 1993). This large number is due to the efficient and diversified sampling methods, and the number of field visits. Among the collected groups, Coleoptera was the most abundant with a rate of 47 %. In this study, we were interested in the order of Coleoptera as bioindicators to detect the quality of environments, as this group is the most widely used in the management of forest ecosystems due to their sensitivity to changes in the state of the environment (Brustel 2001). They were followed by Diptera with 30 %. Pioneer species of Diptera seem to be attracted by the odor emitted by the contents of the traps, particularly the corpses of drowned insects. Hymenoptera occupied the third position, or 17 %. This abundance was due to the great diversity of life and habits of this order (for example ants, wasps, bees, and bumblebees). Hymenoptera formed one of the most abundant groups, being specifically rich and ecologically diverse in temperate regions (Arnan et al 2011). The 14 other remaining orders represent only 6 % of the specimens recorded.
In terms of species richness, Tenebrionidae and Carabidae were the dominant families, each comprising 27 species. Curculionidae, Scarabaeidae, Chrysomelidae, Buprestidae, and Dermestidae followed with 24, 21, 18, 14, and 13 species, respectively. The high diversity of Tenebrionidae aligns with previous studies highlighting their endemism in Morocco (Chavanon 2003, Benyahia et al 2015). Interestingly, eight families were represented by singletons, suggesting potential limitations in trap design or specialized ecological requirements of these species.
Capture rates of Coleoptera were highest in spring (March, April, and May) 2021 (68 %) and early summer (April, May, and June) 2022 (over 87 %), suggesting strong seasonal patterns. These fluctuations were likely linked to the influence of climate variability on vegetation dynamics. Phytophagous, anthophagous, and mycophagous species showed peak abundance during periods of favorable vegetation growth, while detritivores were more prevalent during drier periods (Cardwell et al 1994, Standberg et al 2005, El Harche et al 2021, Buse et al 2015).
Our inventory recorded 256 Coleoptera species, indicating a high level of diversity compared to previous studies on thuya Tetraclinis articulata (Vahl) Mast. (157 species), Iliçaie Quercus ilex L. (206 species), cedar Cedrus atlantica Manetti ex Endl. (268 species), and western Rif fir Abies pisapo maroccana Trab. (540 species) forests (Mouna and Arahou 1986, Arahou 2008, Mouna 2013, Benyahia 2016). Deciduous forests supported a greater number of species than coniferous forests, with more species using various plant parts, such as buds, leaves and wood (Arahou 2008). The higher diversity in deciduous forests may be attributed to a greater variety of ecological niches.
The high biodiversity, as indicated by Shannon indices exceeding four and equitability values between 61 % and 66 %, highlights the rich and complex beetle community in the Maamora cork oak forest. The difference in biodiversity between the stations is influenced by the degree of protection of the plots. It was found that Sstation 2 (highly protected) harbors a more diverse population of beetles than Station 3 (unprotected), which harbored the lowest diversity. This reflects the anthropogenic pressure on this canton, particularly overgrazing. This exceptional biodiversity underscores the ecological and heritage value of this ecosystem. To conserve this valuable resource, it is crucial to reduce threats such as logging and overgrazing, strengthen forest monitoring, and raise public awareness. Sustainable management should consider all aspects of the forest, including ecological, economic, and social values.
A comparative analysis of the three stations indicated a moderate level of similarity (42 %), with variations in species composition attributed to differences in climatic conditions and levels of disturbance. The presence of cork oak as the dominant tree species resulted in a shared core assemblage, although the distribution of functional groups varied across the stations (fig. 7). The state of degradation and density of the vegetation cover is reflected in a precise distribution of functional groups according to their needs (fig. 7). For example, canton E is characterized by the presence of anthophagous and xylophages, due to the large clearings in this canton covered by herbaceous plants that attract pollinators with their blooms. Additionally, the presence of old trees with large cavities provides a favorable habitat for xylophages (such as Scolytidae beetles). Canton C, on the other hand, is a favorable environment for saproxylophagous and necrophages as it is enclosed and reserved for hunting. The presence of a large quantity of dead wood creates a favorable environment for saproxylic species, and there are also carcasses of animals (rabbits, common pheasants, red-legged partridges, etc.). However, canton A is home to diverse functional groups (coprophages, zoophages, phytophagous, and mycophagous). This canton is characterized by a humid climate that favors plant development for much of the year, thereby promoting the presence of mycophagous and phytophagous species. Because they are considered prey for zoophages, this group will proliferate in this area. This canton is also characterized by intense grazing activity, which favors the presence of coprophages.
The diversity of Coleoptera in this ecosystem is reflected in the variety of diets of the recorded species and consequently in their functional role within this ecosystem. The saproxylophagous group is the most diverse (24 %) and the most abundant (41 %); this being well justified by high level of decomposing organic matter, which is essential for both feeding and the completion of their life cycle (Brustel 2001, Fayt et al 2006). This dominance of one group over another may be partly linked to the the fact that the period of activity of the species is associated with their reproductive cycle, which allows them to be active (El Harche et al 2021). The next most abundant group was the phytophagous species, with 21 % of the species recorded. This is not surprising considering we are in a forest environment where there is a very high level of plant matter that is necessary for the development of this functional group (Arahou 2008). Phytophagous species were less abundant (3.5 % of the total). This is probably due to natural regulation by predatory species (zoophagous) which are both well diversified (18 %) and also abundant (6 %). These latter play a particular role in the proper functioning and ecological balance of forest ecosystems. The anthophagous group was poorly represented in terms of species richness (14 %) but they are highly abundant (41 %) thanks to the large areas covered with very diverse and abundant herbaceous plants that that attract flower-feeding beetles. The groups of necrophagous and coprophagous were poorly diversified and low in abundance, which is explained by the low quantity of animal carcasses and organic matter of fecal origin from cattle and sheep that roam the forest, so the grazing pressure in these three stations remains low. These functional groups play an essential role in the mechanisms of decomposition and recycling of dead organic matter, and in soil fertilization (Haloti et al 2006). Among the least represented groups, we find xylophagous and mycophagous species. The xylophagous species develop on living wood and considered forest pests because they feed on the trunks and branches of trees. From an ecological point of view, these species play a very important role in biological diversity and they also promote soil bacterial life. The latter contributes to the natural regeneration of forests, not to mention the importance of xylophagous species in the food chain for many birds that feed on the larvae of Cerambycidae, Buprestidae, Scolytidae and Curculionidae. Mycophagous species regulate the proliferation of fungi in the forest, so this group is found in humid areas (canton A).



















